Reason for Update:
On March 13th, 2020, the Europeans Medicines Agency (EMA) Pharmacovigilance Risk Assessment Committee (PRAC) recommended (Agency 2020):
- Women to stop taking 5-mg ulipristal acetate (UPA) for uterine fibroids while a safety review is ongoing.
- No new patients should start treatment with the medicines, which will be temporarily suspended throughout the European Union (EU) during the review.
On March 18th ,2020, the United Kingdom Medicines and Healthcare products Regulatory Agency issued a similar statement.(Gov.UK 2020)
These decisions were based on a single new case of liver failure and transplant in a 54 year old woman who was being treated with UPA. This occurred despite adherence to the recommended liver function monitoring prior to and during treatment.
Health Canada has been notified of this report by Allergan Canada, the Canadian distributor of Ulipristal Acetate (brand name Fibristal). The complete Canadian safety data that is available to date has also been submitted by Allergan.
Historical Context
Ulipristal Acetate was commercially launched internationally as Esmya (UPA 5mg) in February 2012 and in October 2013 as Fibristal (UPA 5mg) in Canada. It is currently indicated for:
- Preoperative treatment of moderate to severe signs and symptoms of uterine fibroids in adult women of reproductive age.
- Intermittent treatment of moderate to severe signs and symptoms of uterine fibroids in adult women of reproductive age who are not eligible for surgery.
In the original clinical development program, there were no indications of risk of liver injury (Donnez, et al. 2018). It is important to note that patients were excluded from all trials for UPA if they had a baseline abnormality in their liver function or were known to be alcohol dependent.
Internationally, as of January 2020, over 927 800 women have been treated with UPA 5mg. (Gideon Richter)
In Canada, as of Feburary 29th, 2020, there have been over 261 000 prescriptions written since the approval of Fibristal with an estimate of over 120 000 individuals treated nationally. This does not include medication distributed through the sample program or compassionate care program. (Communication with Allergan Canada March 16, 2020).
Initial Safety Notice
On December 1, 2017, the EMA announced that it was conducting a review of UPA 5mg due to 3 reported cases of liver transplantation in patients who were exposed to UPA and another case discovered during the review. The EMA completed its review in May 2018 and outlined guidance for UPA use and the need for hepatic monitoring.
Health Canada commenced its own review in March 2018 and completed its review in December 2018. Health Canada provided an update in Jan 2019 and stated that there may be a link between UPA and risk of serious liver injury. (H. Canada 2019)
The Fibristal Canadian product monograph was updated most recently in July 2019 and the SOGC Guideline on the Medical Management of Uterine Fibroids in October 2019. (A. Canada 2019) (Laberge 2019)
Current Guidance
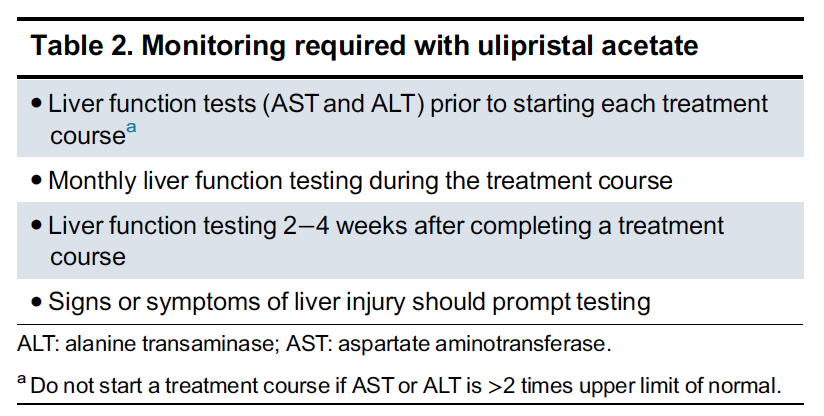
Ulipristal acetate has been showed to be effective in decreasing heavy menstrual bleeding and improving quality of life in women with symptomatic fibroids. However, gynecologists should be aware that there is a risk of severe liver injury associated with ulipristal acetate use. It is important that health care providers follow the guidance provided in the product monograph, and as summarized in the SOGC guideline update. (See Figure 1).
The key considerations include:
- Patient counselling regarding a potential risk, the need for baseline liver testing (ALT, AST) and education regarding the development of symptoms of liver injury while on therapy
◦ Patient compliance to monitoring is essential to continue therapy and cessation of therapy should be considered among patients unable to access testing
- Evaluation of patient history of potential of underlying liver compromise or concomitant medication or substance use that may potentiate risk
- Stop therapy with [UPA] if there is an elevation of transaminase levels (ALT or AST) > 3 times the upper limit of normal followed by full evaluation
At this point there is no change to prescribing or changes to our practice, however, the SOGC and CanSAGE will notify membership of changes as they are updated and disseminated by Health Canada.
Figure 1: Monitoring guidance as per Laberge et al (Laberge 2019)
Bibliography
Agency, European Medicines. 2020. EMA. March 13. Accessed March 20, 2020. https://www.ema.europa.eu/en/medicines/human/referrals/ulipristal-acetate-5mg-medicinal-products.
Canada, Allergan. 2019. "Fibristal Product Monography." July 3. Accessed March 29, 2020. https://allergan-web-cdn-prod.azureedge.net/allergancanadaspecialty/allergancanadaspecialty/media/actavis-canada-specialty/en/products/pms/2019-07-03-fibristal-pm-english.pdf.
Canada, Health. 2019. Health Canada Safety Review Ulipristal Acetate. January 11. Accessed March 29, 2020. https://healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2019/68806a-eng.php.
Donnez, J, P Arriagada, M Marciniak, and D Larrey. 2018. "Liver safety parameters of ulipristal acetate for the treatment of uterine fibroids: a comprehensive review of the clinical development program." EXPERT OPINION ON DRUG SAFETY 1225–1232.
2020. Gov.UK. march 18. Accessed march 29, 2020. https://www.gov.uk/drug-safety-update/esmya-ulipristal-acetate-suspension-of-the-licence-due-to-risk-of-serious-liver-injury.
Laberge, P. 2019. "Guideline No. 389-Medical Management of Symptomatic Uterine Leiomyomas − An Addendum." Journal of Obstetrics and Gynecology Canada.